Abstract
Somatic mutations in the ER chaperone calreticulin (CALR) are frequent and disease-initiating in myeloproliferative neoplasms (MPN). Although the mechanism of mutant CALR-induced MPN is known to involve pathogenic binding between mutant CALR and MPL, this insight has not yet been exploited therapeutically. Consequently, a major deficiency is the lack of clonally selective therapeutic agents with curative potential. Hence, we set out to discover and validate unique genetic dependencies for mutant CALR-driven oncogenesis.
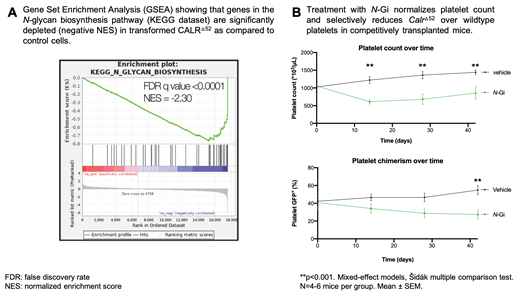
We first performed a whole-genome CRISPR knockout screen in CALR Δ52 MPL-expressing hematopoietic cells to identify genes that were differentially required for the growth of cytokine-independent, transformed CALR Δ52 cells as compared to control cells. Using gene-set enrichment analyses, we identified the N-glycan biosynthesis, unfolded protein response, and the protein secretion pathways to be amongst the most significantly differentially depleted pathways (FDR q values <0.001, 0.014, and 0.025, respectively) in CALR Δ52 cells. We performed a secondary CRISPR pooled screen focused on significant pathways from the primary screen and confirmed these findings.
Strikingly, seven of the top ten hits in both screens were linked to protein N-glycosylation. Four of those genes encode proteins involved in the enzymatic activity of dolichol-phosphate mannose synthase (DPM1, DPM2, DPM3, and MPDU1). This enzyme synthesizes dolichol D-mannosyl phosphate, an essential substrate for protein N-glycosylation. Importantly, these findings from an unbiased whole-genome screen align with prior mechanistic studies demonstrating that both the N-glycosylation sites on MPL and the lectin-binding sites on CALR Δ52 are required for mutant CALR-driven oncogenesis.
We next performed single gene CRISPR Cas9 validation studies and found that DPM2 is required for CALR Δ52-mediated transformation, as demonstrated by increased cell death, reduced p-STAT5 and decreased MPL cell-surface levels, when Dpm2 is knocked out. Importantly, cells cultured in cytokine-rich medium were unaffected by DPM2 loss. Upon cytokine withdrawal, a sub-clone of non-edited Dpm2WT CALR Δ52 cells grew out, further demonstrating requirement for DPM2 for the survival of CALR Δ52 cells. Additionally, we observed a >50% reduction in ex vivo myeloid colony formation of murine CalrΔ52 Dpm2 ko bone marrow (BM) compared with CRISPR-Cas9 non-targeting controls, with non-significant effects on CalrWT BM cells.
To enable clinical translation, we performed a pharmacological screen targeting pathways significantly depleted in our CRISPR screens. Screening 70 drugs, we found that the N-glycosylation pathway was the only pathway in which all tested compounds preferentially killed CALR Δ52 transformed cells.
We then treated primary Calr Δ52/+ mice with a clinical grade N-glycosylation (N-Gi) inhibitor and found platelet counts (Sysmex) to be significantly reduced (vehicle 3x10 6/mL, N-Gi 1x10 6/mL after 18 days, p<.0001). Concordantly, the proportion of megakaryocyte erythrocyte progenitors (MEPs) was significantly reduced in CalrΔ52 BM (p=0.03).
We next performed competitive BM transplantation assays using CD45.2 UBC-GFP MxCre CalrΔ52 knockin and CD45.1 mice. We found that mice treated with N-Gi had significantly reduced platelet counts (vehicle 1440x10 6/mL, N-Gi 845x10 6/mL, p=0.005) as well as significantly reduced platelet chimerism (vehicle 55%, N-Gi 27%, p<0.001), indicating a distinct vulnerability of CalrΔ52 over WT cells.
Finally, we interrogated RNA-sequencing data from primary human MPN platelets. We found N-glycosylation-related pathways to be significantly upregulated in CALR-mutated platelets (n = 13) compared to healthy control platelets (n = 21), highlighting the relevance of our findings to human MPN.
In summary, using unbiased genetic and focused pharmacological screens, we identified the N-glycan biosynthesis pathway as essential for mutant CALR-driven oncogenesis. Using a pre-clinical MPN model, we found that in vivo inhibition of N-glycosylation normalizes key features of MPN and preferentially targets CalrΔ52 over WT cells. These findings have therapeutic implications through inhibiting N-glycosylation alone or in combination with other agents to advance the development of clonally selective therapeutic approaches in CALR-mutant MPN.
AEM and JSJ contributed equally.
Mullally: Janssen, PharmaEssentia, Constellation and Relay Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal